![]()
Daniel León-Alvarez*, Viviana Patricia Reyes-Gómez, Michael J. Wynne, María Edith Ponce-Márquez and Nataly Quiróz-González
DOI 10.1515/bot-2017-0020
Received 2 March, 2017; accepted 21 April, 2017
Abstract: The morphology of the crustose brown alga Hapalospongidion gelatinosum is described in detail based on specimens collected on the Mexican tropical Pacific coast. Molecular phylogenetic analysis of DNA sequences of chloroplast-encoded RuBisCo large subunit (rbcL) and mitochondrial-encoded cytochrome c oxidase subunit 1 (Cox-1) genes, reveals that our samples form a newly rec- ognized clade distinct and distant from the clades of other families in Ralfsiales. Contrary to the previously proposed synonymy between Hapalospongidion and Mesospora, we conclude that both are distinct genera. We also provide a different interpretation of a diagnostic character that allows a distinction of Basispora from the latter two genera. We propose the erection of the new family Hapalospongidi- aceae to accommodate the sole genus Hapalospongidion. A Lectotype and an Epitype for H. gelatinosum are designated.
Keywords: Hapalospongidiaceae fam. nov.; Hapalospon- gidion gelatinosum; Phaeophyceae; phylogeny; taxonomy.
![]()
*Corresponding author: Daniel León-Alvarez, Departamento de Biología Comparada, Laboratorio de Ficología and Sección de
Algas del Herbario de la Facultad de Ciencias, Universidad Nacional Autónoma de México, UNAM, Ciudad Universitaria, Ciudad de México 04510, Mexico, e-mail: dla@ciencias.unam.mx
Viviana Patricia Reyes-Gómez, and Nataly Quiróz-González: Laboratorio de Ficología and Sección de Algas del Herbario de la Facultad de Ciencias, Universidad Nacional Autónoma de México, UNAM, Ciudad de México 04510, Mexico
María Edith Ponce-Márquez: Laboratorio de Ficología and Sección de Algas del Herbario de la Facultad de Ciencias, Universidad Nacional Autónoma de México, UNAM, Ciudad de México 04510, Mexico;
and Departamento de Biología Comparada, Taller Protistas y Algas, Facultad de Ciencias, Universidad Nacional Autónoma de México, Ciudad Universitaria, Coyoacán 04510, Ciudad de México, Mexico Michael J. Wynne: University of Michigan Herbarium, 3600 Varsity Drive, Ann Arbor, MI 48108, USA; and Department of Ecology and Evolutionary Biologyand Herbarium, University of Michigan, Ann Arbor, MI 48109, USA
Hapalospongidion gelatinosum D.A. Saunders (Ralf- siales, Mesosporaceae) is a mucilaginous, crustose brown alga that grows along the North American Pacific coast on rocks that are exposed at low tide in the litto- ral fringe. It was originally recorded near the Hopkins Marine Station, Pacific Grove (California, USA) by Saun- ders (1899) and later studied by Hollenberg from several places in Carmel Beach, Corona del Mar (California) and from Pacific Mexico from Punta Banda (Baja Cali- fornia) southward to Bahía Petatlán (Guerrero) (Hol- lenberg 1942). However, the last record from California was by Smith at Point Aulon (1944). Since that time, the species has not been seen again at or near the type local- ity, notwithstanding several efforts to find it (John West, Viviana Reyes, pers. com.). By contrast, during our flo- ristic studies along the Pacific coast of Mexico, we have seen H. gelatinosum many times from Nayarit to Oaxaca (León-Alvarez and González-González 1993, Serviere- Zaragoza 1993, Serviere-Zaragoza et al. 1993, León-Tejera et al. 1993, León-Alvarez 1996).
Hapalospongidion gelatinosum is the type species of the genus established by Saunders (1899). Hapalo- spongidion is recognized by its unbranched vegetative filaments and hairs arising from a basal plate that is two cells in thickness, plurangia formed by transforma- tion of the upper cells of the longer vegetative filaments and by unangia arising from the transformation of the terminal cell or cells of the shorter vegetative filaments (Saunders 1899). Hapalospongidion was included in the Mesosporaceae by Tanaka and Chihara (1982) together with two other mucilaginous genera: Mesospora Weber- van Bosse: type species M. schmidtii Weber-van Bosse (1911) and Basispora D.M. John et G.W. Lawson: type species B. africana D.M. John et G.W. Lawson (1974). The family was characterized by having free erect fila- ments, intercalary plurangia and terminal unangia. The distinction between the three genera, however, was not
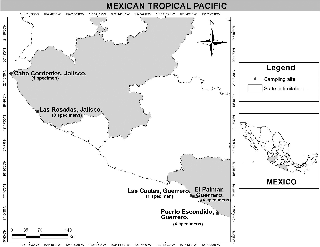
Figure 1: Map of the sampled region.
clarified, and some authors considered the three as taxo- nomic synonyms (Womersley 1987, León-Álvarez and González-González 1993, León-Alvarez 1996, Silva et al. 1996). On the basis of rbcL sequences of several brown crustose algae including Mesospora sp., Lim et al. (2007) showed the Mesosporaceae to be a distinct clade from other families (Ralfsiaceae and Neoralfsiaceae) in the Ralfsiales. However, they did not include specimens of Hapalospongidion in their analyses. In this study, we use the rbcL and Cox-1 genes to clarify the taxonomic status of H. gelatinosum. Also, we characterize the morphology of H. gelatinosum from Mexico in order to understand the relationship between morphological and molecular variations. Our results confirmed that morphological variation corresponds with little molecular variation. On the other hand, our molecular-based studies gave us the unexpected result that Hapalospongidion shows a significant molecular distance from Mesospora in the Mesosporaceae. On the basis of these results, we propose to erect a new family, the Hapalospongidiaceae, which comprises Hapalospongidion as the sole member of the family.
Pieces of rock with gelatinous crusts of brown algae were collected with a chisel and mallet or razor blades, from five localities from the Mexican tropical Pacific coast (Figure 1). Each specimen was observed under the dissecting micro- scope, and material was separated for morphological and molecular analyses. The specimens were dried with paper towels then desiccated in silica gel and stored in a re-seal- able plastic bag at ambient temperature. All samples were incorporated into the Pacifico Tropical Mexicano collection (PTM) of the Algae Section of the Herbarium of the Faculty of Sciences, National Autonomous University of Mexico, FCME. Acronyms of herbaria are according to Thiers (2016).
Light microscope examinations used squash prepa- rations and radial longitudinal sections cut by hand with a razor blade. The preparations were mounted in glyc- erol-gelatin phenol on glass slides. The specimens were observed with an OLYMPUS CX31 compound microscope (Olympus Microscopy, Mexico) and photographed with a Nikon D7000 camera (Nikon, Tokyo, Japan). Images were edited and assembled using Adobe Photoshop version CS6.
Specimens in three herbaria in the U.S.A. were con- sulted: the National Herbarium of the Smithsonian Insti- tution (US), University of California, Berkeley (UC) and Natural History Museum, Los Angeles (LAM, collection now in UC). Small amounts of herbarium specimens were carefully separated from the mica mounts, and slides with glycerol-gelatin phenol were prepared for microscope analysis. The prepared slides were incorporated in the respective collections of these herbaria.
The specimens were identified following the approach taken by León-Alvarez and González-González (1993) and León-Alvarez (1996), and the nomenclature recommended by León-Alvarez and Norris (2005). To obtain precise descriptions regarding the origin of perithallial filaments, the terms primigenous (original filaments) and postigenous (derived filaments) are used in an analogous way to their use by Woelkerling (1988) to describe the dimerous crusts (with postigenous filaments originated by intercalary and trans- verse divisions of primigenous cells) of coralline red algae.
DNA was extracted from 10 to 20 mg of tissue lyophi- lized using DNeasy Plant Mini Kit from Qiagen (Qiagen, Germany), following the manufacturer’s instructions, increasing the incubation time to 60 min. To verify that the extraction process was successful, a volume of 5 μl DNA obtained was checked by electrophoresis for 30 min at 100 volts on 1% agarose gels stained with Biotium GelRed.
The rbcL region was amplified by PCR using the primers rbc-F0, rbc-F3 (Kawai and Sasaki 2004), Ral-R952 (Lim et al. 2007) and PRB-R3 (Kogame et al. 1999) in a Flexigene Techne thermocycler, with Taq PCR Core Kit (Qiagen, Hilden, Germany). The final volume for the PCR reaction was 25 μl:
2.5 μl 10X buffer, 1 μl BSA, 1 μl MgCl2, 0.5 μl dNTP, 0.125 μl Taq DNA polymerase, 1 μl primer F, 1 μl primer R, l μl DNA and 16.875 μl H2O. The parameters of the PCR amplification
were those described by Lim et al. (2007). The Cox-1 gene
was amplified by PCR using the primers GWSLF2 CAAAT- CATAAAGATATCGGCAC and GWSRx ACTTCTGGATGTCC- CGAGAATCA (Gary W. Saunders, unpublished), using a final volume for the PCR reaction of 15 μl: 1.5 μl 10X buffer, 1 μl BSA, 1 μl MgCl2, 0.5 μl dNTP, 0.125 μl Taq DNA polymerase,
0.5 μl primer F, 0.5 μl primer R, l μl DNA and 8.875 μl H2O.
The PCR routine used an initial denaturation for 2 min at
94°C followed by five cycles of denaturation at 94°C for 30 s, annealing at 45°C for 30 s, extension at 72°C for 1 min; fol- lowed by 35 cycles of denaturation at 94°C for 30 s, anneal- ing at 46.5°C for 30 s, extension at 72°C for 1 min; and a final extension at 72°C for 7 min. The PCR products were checked for length and yield by electrophoresis on 1% agarose gels stained with Biotium GelRed and purified with Sephadex® (Sigma-Aldrich, USA). The PCR products were sequenced at
the Molecular Biology Laboratory of the Institute of Biology, Universidad Nacional Autónoma de México (UNAM).
The sequences were edited in the programs Bioedit (Hall 1999) and Sequencher version 4.1.4 sequence analy- sis software (Genes Code Corporation, Ann Arbor, MI USA http://www.genecodes.com). The alignment was done with Mafft v7.158b (Kazutaka and Daron 2013) with 52 rbcL sequences and 35 Cox-1 sequence from the Ralfsiales, with Tilopteris mertensii (Turner) Kützing as an outgroup species. The gene sequences were either newly generated or retrieved from GenBank (Supplemental Table S1).
To evaluate the level of intra- and interspecific vari- ation in the rbcL and Cox-1 sequence, uncorrected (“p”) pairwise genetic distances were estimated using PAUP* 4.0b10 (Swofford 2003). Prior to maximum likelihood (ML) and Bayesian inference (BI), we used the Model Test v. 3.7 program (Posada and Crandall 1998) to find the best model of nucleotide substitution, using the Akaike information criterion (AIC). The model selected for rbcL and Cox-1 data sets was GTR + I + G (general-time-reversible + proportion of invariable sites + gamma distribution). The maximum like- lihood analysis was performed using the program RaxML (Randomized Accelerated Maximum Likelihood) (Stama- takis et al. 2012), with 1000 random sequences under the heuristic search algorithm and 1000 bootstrap replicates.
The Bayesian analysis was performed using the program MrBayes 3.2.2 (Huelsenbeck and Ronquist 2001), using four chains of the Markov chain Monte Carlo runs, sampling one tree every 1000 generations for 10 millions generations, starting with a random tree. The analysis was concluded when the average deviation of split frequencies was less than 0.01, this was determined using Tracer v.
1.5 (http://tree.bio.ed.ac.uk/software/tracer/) to ensure that stationarity was reached and to determine a suitable burn-in value. We discarded the first 1,000,000 genera- tions in both runs as the burn-in was 25% (2.5 million gen- erations) to build the consensus tree.
Saunders (1899) did not designate a holotype of Hapalo- spongidion gelatinosum in the protologue. We have seen part of the original material of the species, and we have assigned a lectotype whose morphology is described below: Hapalospongidion gelatinosum Saunders 1899: 37–38,
Pl. I, figs 1, 2, 3, 3, 4, 4′, 4′′ and 4′′′.
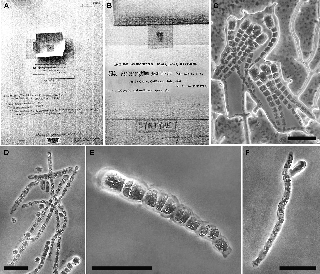
Figure 2: Hapalospongidion gelatinosum Lectotype.
(A–B). US56545 sheet with the original material of H. gelatinosum in mica from Phycotheca Boreali-Americana No. 534 and later hand- written annotations. (C) Free unbranched erect filaments increasing in diameter towards their distal ends, which have enlarged terminal dome-shaped cells filled with pigments. (D) Probable lateral unangia (upper filament). (E) Probable plurangium. (F) Terminal, lateral and presumably abortive unangia. Scale bars = 50 μm.
US56545, (Phycotheca Boreali-Americana by Collins, Holden and Setchell No. 534). One slide prepared by
D. León from original material in mica. This lecto- type herbarium sheet in US (Figures 2A and B) bears later hand-written annotations referring to Saunders’ (1899) publication. This is part of the original material that was collected by Saunders at Mussel Point (=Point Aulon = Laboratory Point), the Hopkins Marine Station at Pacific Grove, California, USA and then distributed with the number 534 by Collins, Holden and Setchell as a part of “Phycotheca Boreali-Americana” (PB-A) to
numerous herbaria including BRU, F, GMS, MICH, MU, NY, UC and US. Saunders did not cite the PB-A 534 in the 1899 description. The sets of PB-A 534 were distributed as little pieces of material adhered to mica, and all the speci- mens seemed to have at least some of the characters that are recognizable in the Saunders description (1899), such as light honey-brown colour when wet, freely expanded, unbranched erect filaments covered by mucilage, with 24–31 cells, 387–477 μm in length, increasing slightly in diameter toward their distal ends, which have large ter- minal dome-shaped cells (17–27 μm length × 9.4–20 μm diameter) filled with pigments, and identified as dubious plurangia and unangia; the latter are probably abortive (Figures 2C–F).
The Lectotype of Hapalospongidion gelatinosum lacks some of the characters to be certain of the identity of the species (basal layer, phaeophycean hairs and indubitable reproductive structures). To have an unambiguous appli- cation of the name, we have designated an epitype that has all of the characters described by Saunders in his orig- inal description of the species (1899). These characters are included in the following description:
PTM10056 in FCME, UNAM. Cabo Corrientes, Jal. Apr. 16, 2015, coll. V. Reyes. Slide 1964, with unangia. Lectotype US56545 (above). Isoepitypes in MICH, UC and US.
PTM9622, 9627, 9657, 9576, 9663, 9665, 9666, 9667, 9668, 10056, 10085, 10089 and 10091 all in the PTM collection in FCME.
AHFH72247 in UC1832934 (after LAM599704, coll. Dawson in Cabeza de Ballena, near Cabo San Lucas, Baja California Sur, Mexico, 1946, det. G.J. Hollenberg); AHFH48147 in UC1832935 (after LAM599701, first headland south of Punta Santa Rosalía, B.C., coll. E.Y. Dawson, det. Hollenberg, honey-brown, thick crust-like Eu-ralfsia-), AHFH 48146 in UC1832933 (after LAM599702, between Cabo Pulmo and Punta Frailes, B.C., coll. E.Y. Dawson, det. Hollenberg, as green circular dot).
Crustose thalli, with conspicuous rounded margins and lobes growing extensively and firmly attached to the substrate, without rhizoids, spongiose, without evident growth lines, light honey-brown to dark green when wet and dark to coppery brown when dried, embossed and slippery surfaces, mucilaginous (Figures 3A and B). Thalli 230–850 (1100) μm thick, constructed of free, erect, mostly unbranched postigenous filaments (Figures 3C and D) that are borne transversely from intercalary primigenous cells or arise creeping at acute angles from the terminal apical cells of the primigenous filaments. Primigenous filaments form 2–3 prostrate basal layers, composed of cells 10–15 (30) μm long, 4–10 (13) μm high and irregular in shape (Figures 3D–F). Postigenous filaments are 220–830 (1050) μm long, slightly increasing distally in diameter while its cells decrease in length (but sometimes increase), composed of 17–52 (72) cells that are cylindrical in the basal part, 12–20
(33) μm long and 6–8 (11) μm wide; cells in the middle part of postigenous filaments are cylindrical, 10–20 μm long and 6–8 μm wide; cells in upper part (2–3 cells below the apical cell) are doliiform, 8–10 (15) μm long and (5) 8–10 (12)
μm wide; apical cells are variable in shape (dome, obovoid or club-shaped), (8) 10–15 μm long and 8–12 (14) μm wide (Figures 4A and B). Single irregularly shaped plastid per cell in mid to apical part of postigenous filaments, one to several dubious discoidal plastids in basal layers. Phaeophycean hairs irregularly distributed in the thallus, arising from the base or in the middle part of erect filaments, alone or in groups (Figure 4C). Unangial reproductive thalli produce filaments of two types: both originated from the second or third postigenous cell that branches to give rise to a spindly filament (paraphysis-like) and another thicker and shorter filament (stalk-like), which supports a terminal unangium. These spindly paraphysis-like filaments were morphologi- cally identical to vegetative ones (Figures 4D and E). The unangia have an obovoid-shape, 55–83 (125) μm long and 20–33 (41) μm wide. Stalk-like filaments have 7–19 (31) cells, 10–23 μm long and 10–20 μm wide in the basal part of stalk, and 7–16 μm and 8–13 (15) μm in the apical part (Figure 4E). Unangia were rare, present only in 2 of 14 specimens. In plu- rangial thalli all vegetative and reproductive filaments are similar in length. Cells from the upper part of the postige- nous filaments become transformed to cylindrical, swollen plurangia, 22–52 (75) μm long, 10–18 (28) μm wide with 1–7 terminal sterile cells. Plurangia have uniseriate or biseriate irregularly arranged loci (Figure 4F).
The rbcL sequence alignment included 52 taxa belonging to the order Ralfsiales Nakamura ex Lim et Kawai and was 1300 bp in length. The two methods of phylogenetic infer- ence (ML, BI) showed trees with identical topologies, rooted by Tilopteris mertensii. Four major groups were recognized with moderately to highly supported Bootstrap values (BP) and Bayesian posterior probability (PP; Figure 5). The group comprising specimens of Hapalospongidion gelatino- sum (Clade III) was highly supported (100/100).
The Cox-1 sequence alignment included 35 taxa belonging to order Ralfsiales Nakamura ex Lim et Kawai and was 604 bp in length. The two methods of phyloge- netic inference (ML, BI) showed congruent but not identi- cal trees, rooted by T. mertensii. Four major groups were recognized with well to highly supported Bootstrap values and Bayesian posterior probability (Figure 6). Intra- and interspecific divergences (%) for rbcL and Cox-1 genes were compared between H. gelatinosum and the species of Mesospora, Neoralfsia and Ralfsia (Table 1). Divergences between species of Mesosporaceae, Ralfsiaceae and Neoralfsiaceae are given in supplementary data (Supple- mental Table S2).
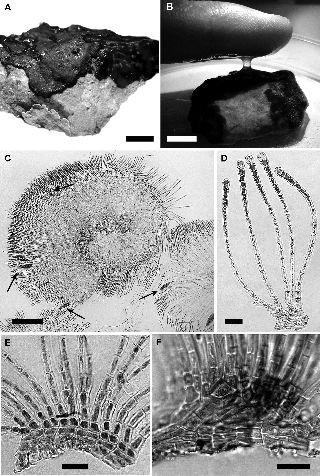
Figure 3: Hapalospongidion gelatinosum from Pacific Mexico.
(A) Irregular margin of the growing crust, scale bar = 1 cm (PTM9663). (B) Adherent mucilage after touching the crust in wet condition, scale bar = 1 cm., (PTM10085). (C) Squashed reproductive unangial specimen (arrows), scale bar = 100 μm (PTM10056). (D–F) Free vegetative unbranched postigenous filaments arising at different angles from the primigenous basal layers; (D) transverse or slightly acute angle, scale bar = 25 μm (PTM10056); (E) transverse angle, scale bar = 20 μm (PTM10056); (F) acute angle, scale bar = 50 μm (PTM9665).
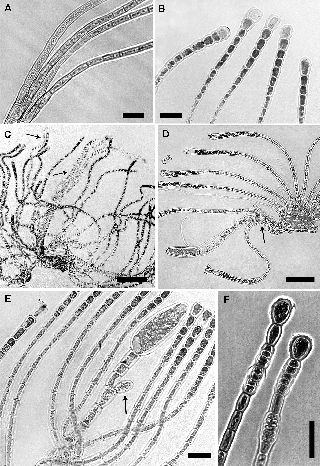
Figure 4: Hapalospongidion gelatinosum from Pacific Mexico.
(A–B) Morphological variation of the vegetative postigenous filaments in mid and apical part, scale bars = 50 μm and 20 μm, respec- tively (PTM9657, 10056). (C) Phaeophycean hairs arising at base or mid-region of the postigenous filaments (arrows), scale bar = 200 μm (PTM10085). (D) Postigenous dividing cell that also produces a paraphysis-like filament or similar vegetative filament (arrow), scale bar = 50 μm (PTM10056). (E) Possible abortive unangium (arrow), scale bar = 20 μm (PTM10056). (F) Different types of swollen intercalary plurangia under 1-2 sterile cells, scale bar = 50 μm (PTM9627).
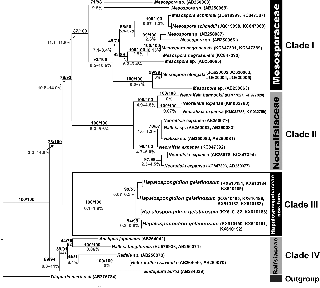
Figure 5: rbcL consensus tree with maximum likelihood/Bayesian inference (ML/BI) supported values of clade (above) and range of uncor- rected distances between clades (%) (below).
The small amount of original material in the PB-A 534 col- lection made it difficult to make observations and was inad- equate for molecular studies. However, we could observe in Lectotype US56545 the general vegetative morphology, doubtful “abortive” unangia and plurangia (Figures 2E and F). However, we could not see any mature reproductive structures with certainty, even though unangia and pluran- gia were indicated in the PB-A 534 labelled specimens. The erect filaments with large terminal cells filled with pigment could be unangia in an early stage of development (Figures 2C and D), but we think that they are better interpreted as apical vegetative cells with a cortical function. In the Mexican specimens we have seen similar large terminal cells, but these are smaller than in the PB-A 534 specimens.
J.A. West, K.A. Miller and R.L. Moe searched for Hapa- lospongidion gelatinosum in Monterey, California, in April 2013, but they were unable to find it (J. West pers. comm.), despite previous reports that the species was common (Hollenberg 1942, Smith 1944). We went to the type locality at the Hopkins Marine Station and surrounding localities (Monterey Peninsula: Pebble Beach, China Rock, Carmel Beach; Santa Cruz: Mitchell’s Cove in West Cliff, Pleas- ure Point and Dog Beach) with the specific goal to collect the species (17 November to 19 December 2014), but we were also unsuccessful. We consider our Mexican Tropi- cal Pacific material representative of the species because it corresponds with the original description by Saunders (1899) and because the study done by Hollenberg (1942) included specimens from the type locality and from the Pacific coast of Mexico (south to Guerrero).
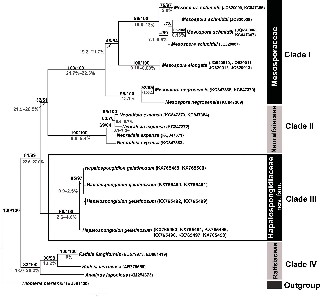
Figure 6: Cox-1 consensus tree with maximum likelihood/Bayesian inference (ML/BI) supported values of clade (above) and range of uncor- rected distances between clades (%) (below).
Table 1: Intra- and interspecific divergence (%) of rbcL and Cox-1 genes between Hapalospongidion gelatinosum and remaining species of Ralfsiales.
![]()
![]()
![]()
Species rbcL % divergence Cox-1 % divergence
H. gelatinosum | H. gelatinosum | 0–1.8% | 0–4.6% |
H. gelatinosum | Mesospora schmidtii | 11.7–12.9% | 21.3–23.2% |
H. gelatinosum | M. elongata | 11.0–13.0% | 21.6–25.0% |
H. gelatinosum | M. negrosensis | 11.6–13.1% | 24.4–27.0% |
H. gelatinosum | Neoralfsia expansa sensu Lim et al. (2007) | 12.1–13.5% | 21.0–23.4% |
H. gelatinosum | Neoralfsia expansa | 13.7–14.8% | – |
H. gelatinosum | Neoralfsia hancockii | 13.6–14.6% | – |
H. gelatinosum | Heteroralfsia saxicola | 12.3–13.5% | – |
H. gelatinosum | Endoplura aurea | 12.6–13.1% | – |
H. gelatinosum | Ralfsia fungiformis | 11.4–12.6% | 20.0–21.4% |
H. gelatinosum | Ralfsia verrucosa | – | 20.1–20.7% |
H. gelatinosum | Analipus japonicus | 11.1–12.2% | 20.2–21.6% |
Table 2: Morphological comparison between Hapalospongidion gelatinosum, Mesospora species and Basispora africana.
![]()
H. gelatinosum (Saunders 1899) M. schmidtii (Weber van-Bosse 1911, Poong et al. 2013)
M. negrosensis (West and Calumpong 1996)
M. elongata (Poong et al.
2013)
Basispora africana (John and Lawson 1974)
![]()
Type locality California, USA Indonesia Lalaan, Philippines Okinawa, Japan Ghana Thickness 230-850 (1100) μm Up to 350 μm Up to 200 μm ? ?
Number of layers of primigenous filaments
Cell number of postigenous filament
2 (up to 4) (1)–2–(4) 2–3 ? 1-several 17–52 (72) 10–19 Up to 20 11–29 22
Phaeophycean hairs Emerging as tufts in the middle of vegetative filaments
Emerging as tufts from depressions in the basal layer, three times longer than vegetative filaments
Emerging from the basal layer, two times longer than vegetative filaments
Hair tufts were occasionally ?
observed
Brought to you by | Universidad Nacional Autonoma
Authenticated | dla@ciencias.unam.mx author's copy Download Date | 6/3/17 2:38 AM
Basal cell of the postigenous filament: length/diameter
12–20 (33) μm/6–8 (11) μm 7.2–14.8 (17.7) μm/2.8–6.8 (10.0) μm
11–15 μm/ 8–10 μm 6.3–15.9 (–23.7) μm/
2.5–6.0 (–9.2) μm
Larger than diameter/2.5–5 μm
Apical cell of the postigenous filament: length/diameter
(8) 10–15 μm/8–12 (14) μm 7–11 (–18)/7–12 μm ?/11–13 μm 5.8–10.7(17.1)/3.4–7.9 (13.2) μm
?/8–11 μm
Unangia position Terminally inserted on stalks, lateral to paraphysis-like filament
Terminally inserted on stalks, lateral and basal to the surrounding filaments
? Terminally inserted on stalks, lateral and basal to the surrounding filaments
Terminally inserted on stalks, lateral and basal to the surrounding filaments
Unangium: length/ diameter
55–83 (125) μm/20–33 (41) μm 63.3–143.2 μm/20.2–48.8 μm – (4.8) 8.1–10.9 μm/11.5–26.9
μm
70–106 (125)/15–33 (45)
D. León-Alvarez et al.: Hapalospongidion gelatinosum, Hapalospongidiaceae fam. nov.
Stalk cells number 7–19 (31) cells Up to 4 cells – Up to 8 cell (4) 6–10 (15)
Plurangia Subterminal clavate, elongate swollen shape
Intercalary Intercalary ? ?
Plurangia: length/diameter 22–52 (75) μm/10–18 (28) μm 23–34 (43)/6.6–10 (12.3) μm ? 22.1–50 μm/(5) 8.1–11 μm –
![]()
?, not known; –, does not apply.
The morphological variation in H. gelatinosum has been included in the Epitype description, and some char- acters are indicated (Figures 3 and 4). Our results show that, in spite of morphological variation, molecular vari- ation is low (rbcL 0–1.8%, Cox-1 0–4.6%, Table 1), as we would expect for a single species.
Based on material of Mesospora schmidtii obtained from the type locality, Poong et al. (2013) argued that Mes- ospora is a genus morphologically distinct from Hapalo- spongidion. They remarked that Mesospora and Basispora (the third genus in the Mesosporaceae) do not have the immature or abortive unangia illustrated by Saunders (1899) as series of enlarged cells. We have not seen such cells, although we have infrequently seen unangia like the abortive organs as drawn by Hollenberg 1942 in H. gelatinosum specimens from Pacific Mexico (Figure 4E). Poong et al. (2013) also distinguished Hapalospongidion as having terminal unangia arising directly from the basal layer instead of their arising on a morphologically differ- entiated stalk cell or cells that issued laterally from erect filaments in Mesospora (and not necessarily in a basal position). Later, Poong (2014) confirmed seeing stalks morphologically differentiated from vegetative erect fila- ments in H. saxigenum. Both our specimens and Saunders’ description have stalk cells morphologically differenti- ated from vegetative ones (as in Mesospora schmidtii). With respect to figure 2 of Saunders (1899) illustrating the terminal unangia arising “directly” from a basal layer, we have a different interpretation. In specimens from México we have seen the unangial filaments arising from a basal branching postigenous cell (first to third postigenous cell), that is, not directly from a basal layer but from a postigenous cell that is cut off from an intercalary primig- enous cell borne on the basal layer. Such a basal branch- ing postigenous cell produces two types of filaments: one reproductive filament generating a terminal unangium and one producing a vegetative paraphysis-like fila- ment. We can see this pattern in M. schmidtii (Tanaka and Chihara 1982, fig. 7), H. pangoense (Setchell) Hollenberg as drawn by Setchell (1924, fig. 33, 3) and in UC221298, (Type from Samoa, Tutuila Island, as Ralfsia pangoensis Setchell), and H. gelatinosum from México. We think the Plate I, figure 2 from Saunders (1899) could show only the unbranched and free erect filaments arising from a “basal mass” two cells thick without any reference to the origin of erect filaments or to the plane of the view (i.e. tangential versus radial view). One variation of this pattern is present in Basispora africana in which the origin of the unangial filament is a branching postigenous cell that branches again, producing two filaments, one of which has the terminal unangium and the other is the paraphysis-like
filament. According to this interpretation, this charac- ter separates only Basispora from Mesopora and Hapa- lospongidion, and this character is identical in the latter two genera. It would be necessary to carry out molecular studies on authentic specimens of Basispora in order to verify its separation from the latter two genera.
Only the thickness of the thallus [230–850 (1100) μm], the number of cells of the postigenous filaments [17–52 (71)] and the number of stalk cells of the unangium [7–19 (31)] in H. gelatinosum can be considered as morphologi- cal characters that distinguish it from Mesospora species (M. schmidtii, M. elongata and M. negrosensis), which are thinner and with shorter stalks (Table 2). Furthermore, molecular results show both genera are clearly distinct (clades I and III, Figures 5 and 6) and distant (rbcL diver- gence 11.0–13.1%, Cox-1 divergence 21.3–27%; Table 1). Similar results were found in Poong’s thesis (2014), where she suggested that Mesospora is a genus distinct from Hapalospongidion based on a molecular sequence of H. saxigenum Lindauer (1974) from New Zealand.
Currently, seven species of Hapalospongidion are recognised around the world (Guiry and Guiry 2017): H. gelatinosum from California (Pacific Grove), H. pangoense (Setchell) Hollenberg (1942) from Samoa, H. saxigenum Lindauer (1974) from New Zealand, H. capitatum Womer- sley (1987) from Australia, H. macrocarpum (Feldmann)
D. León-Alvarez et J. González-González (1993) from Algeria,
H. van-bosseae (Børgesen) D. León-Alvarez et J. González- González (1993) from Easter Island, and H. thirumullavara- mense P. Sophiammal Nettar et M.V.N. Panikkar (2009) from India. Hapalospongidion pangoense and H. macrocarpum can be distinguished from H. gelatinosum by their thinner thalli and shorter stalks (0–5 cells) supporting unangia.
H. thirumullavaramense and H. van-bosseae have several basal layers (9–12 and 9 cell layers, respectively) compared to 2–3 layers in H. gelatinosum, and the plurangia in H. cap- itatum are distinctive (swollen, capitate) and not as seen in
H. gelatinosum (Table 3). According to Buchanan (2005), H. saxigenum is morphologically identical to H. gelatinosum. However, preliminary rbcL analysis appears to show that the species are distinct even to family level (unpublished data). Likewise, it will be necessary to carry out molecular studies with the remaining species of Hapalospongidion to establish their phylogenetic relationships.
Our molecular phylogenetic analyses showed a new clade (Clade III) that we consider to be an independent taxon at the family level, which is clearly distinct at the molecular level (support rbcL 100/100, Cox-1 99/100) and which we have called Hapalospongidiaceae. This clade appears to be sister to another clade shared by the two strongly supported families, the Neoralfsiaceae (rbcL
![]()
![]()
D. León-Alvarez et al.: Hapalospongidion gelatinosum, Hapalospongidiaceae fam. nov.
Brought to you by | Universidad Nacional Autonoma
Authenticated | dla@ciencias.unam.mx author's copy Download Date | 6/3/17 2:38 AM
Table 3: Morphological comparison between Hapalospongidion gelatinosum and other species of Hapalospongidion.
![]()
H. gelatinosum (Saunders 1899) | H. pangoensis (Setchell 1924, Hollenberg 1942, UC221298) | H. van-bosseae (Borgesen 1924) | H. macrocarpum (Feldman 1931) | H. saxigenum (Lindauer 1949) | H. capitatum (Womersley 1987) | H. thirumullavaramense (Sophiammal and Panikkar 2009) | |
Type locality | California, USA | Pagopago Harbor, | Hanga Piko, | Cherchell, Algeria | Stewart Island, | King George | Kerala, India |
Samoa | Easter Island | New Zealand | Sound, Western | ||||
Australia | |||||||
Thickness | 230–850 (1100) μm | 180–225 μm | ? | 400–450 μm | 225–725 μm | 500–700 μm | ? |
Number of layers of | 2 (up to 4) | 2–3 | 10 or more | ? | 2–3 | 2–3 | 9–12 |
primigenous filaments | |||||||
Cell number of | 17–52 (72) | 15–20 | 20–30 | 20–25 | Up to 60 | 40–60 | Up to 85 |
postigenous filaments | |||||||
Phaeophycean hairs | Emerging as tufts in the | ? | Emerging as | ? | Emerging as tufts | ? | ? |
middle of vegetative | tufts below the | in the middle of | |||||
filaments | surface of the | vegetative filaments | |||||
thallus | |||||||
Basal cell of the | 12–20 (33) μm/6–8 | 9–18 μm/6–8.7 μm | 24 μm/8–11 μm | 12–15 μm/10– | 12–36 μm/6–18 μm | 10–18 μm/4–6 μm | 7–16 μm/6–13 μm |
postigenous filament | (11) μm | 12 μm | |||||
length/diameter | |||||||
Apical cell of the | (8) 10–15 μm/8–12 | ? | 24 μm/20 μm | ?/15 μm | ?/6–18 μm | ?/10–12 μm | 7.5–15.0 μm/6.2–12.5 |
postigenous filament | (14) μm | μm | |||||
length/diameter | |||||||
Unangium position | Terminally inserted | Terminally inserted | ? | Laterally to | Terminal | ? | ? |
on stalks, lateral | on stalks, lateral to | paraphyses | |||||
to paraphysis-like | paraphysis-like filament | ||||||
filament | |||||||
Unangia: length/ | 55–83 (125) μm/ | 58–167 μm/30–100 μm | – | 200–300 | 105–170 μm/36–45 | – | – |
diameter | 20–33 (41) μm | μm/60–70 μm | μm | ||||
Stalk cells number | 7–19 (31) | 3–5 | – | 0–2 | 12 | – | – |
Plurangium | Subterminal. Club, | ? | Subterminal. | ? | ? | Terminal, capitate | Intercalary with collar- |
elongate swollen shape | Club, elongate | like lateral projections | |||||
swollen shape | |||||||
Plurangia: length/ | 22–52 (75) μm/10–18 | – | ? | – | – | 40–60 (80) | 28–42 μm/9–15 μm |
diameter | (28) μm | μm/15–20 μm |
and Cox-1 100/100) and the Mesosporaceae (rbcL 87/100, Cox-1 100/100; Figures 5 and 6). However, at present this phylogenetic relationship cannot be verified due to the limited molecular knowledge of the families in the Ralf- siales, which results in reduced values of support above the family level (rbcL 76/93, Cox-1 32/51). The Hapalospon- gidiaceae is morphologically difficult to separate from the Mesosporaceae, but it can be distinguished from the other two families in the order Ralfsiales (Ralfsiaceae and Neoralfsiaceae) by its free erect filaments. More studies will be needed to determine if free erect filaments evolved twice in the Ralfsiales, as families like the Ralfsiaceae and Neoralfsiaceae have connate erect filaments.
Hapalospongidiaceae fam. nov. V. Reyes Gómez et D. León-Alvarez
With the characters of the genus Hapalospongidion
D.A. Saunders. rbcL and Cox-1 sequences form a distinct clade.
Type genus: Hapalospongidion D.A. Saunders (1899). New or little-known brown algae of the Pacific coast. Erythea 7: 37.
Børgesen, F. 1924. Marine algae from Easter Island. In: (C. Skotts- berg, ed.) The Natural History of Juan Fernandez and Easter Island. Vol. 2: Almquist and Wiksells, Uppsala. pp. 247–309.
Buchanan, J. 2005. The crustose brown algae of New Zealand: a taxonomic study. Masters thesis, Victoria University of Wel- lington, New Zealand.
Feldmann, J. 1931. Contribution à la flore algologique marine de l’Algérie. Les algues de Cherchell. Bull. Soc. Hist. Nat. Afr. Nord 22: 179–254.
Guiry, M.D. and G.M. Guiry. 2017. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www. algaebase.org; searched on 2 March 2017.
Hall, T.A. 1999. BioEdit a user-friendly biological sequence align- ment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41: 95–98.
Hollenberg, G.J. 1942. Phycological notes. Bull. Torrey Bot. Club 69: 528–538.
Huelsenbeck, J.P. and F. Ronquist. 2001. Mr. Bayes: Bayesian infer- ence of phylogenetic trees. Bioinformatics 17: 754–755.
John, D.M. and G.W. Lawson. 1974. Basispora, a new genus of the Ralfsiaceae. Br. Phycol. J. 9: 285–290.
Kawai, H. and H. Sasaki. 2004. Morphology, life history, and molecular phylogeny of Stschapovia flagellaris (Tilopteridales, Phaeophyceae) and the erection of the family Stschapoviaceae fam. nov. J. Phycol. 40: 1156–1169.
Kawai, H., T. Hanyuda, S.G.A. Draisma, R.T. Wilce, and R.A. Andersen. 2015. Molecular phylogeny of two unusual brown algae, Phaeostrophion irregulare and Platysiphon glacialis, proposal of the Stschapoviales ord. nov. and Platysiphonaceae fam. nov., and a re-examination of divergence times for brown algal orders. J. Phycol. 51: 918–928.
Kazutaka, K. and M.S. Daron. 2013. MAFFT multiple sequence align- ment software version 7: improvements in performance and usability. Molec. Biol. Evol. 30: 772–780.
Kogame, K., T. Horiguchi, and M. Masuda. 1999. Phylogeny of the order Scytosiphonales (Phaeophyceae) based on DNA sequences of rbcL, partial rbcS, and partial LSU nr DNA. Phyco- logia 38: 496–502.
León-Alvarez, D. 1996. Feofitas costrosas del Pacífico tropical mexi- cano: contribución a la flora tónica de macroalgas de la región. Tesis Doctoral, Facultad de Ciencias, Universidad Nacional Autónoma de México, pp 290.
León-Alvarez, D. and J. González-González. 1993. Algas costrosas del Pacifico Tropical. In: (Salazar-Vallejo, S. I. and González, N. E., eds.) Biodiversidad Marina y Costera de México. Comisión Nacional de Biodiversidad y Centro de Investigaciones de Quin- tana Roo, México, pp. 456–474.
León-Alvarez, D. and J.N. Norris. 2005. Terminology and position of reproductive structures in crustose brown algae: misapplica- tion, confusion and clarification. Cryptogam. Algol. 26: 91–102.
León-Alvarez, D., M.L. Núnez-Resendiz, and M.E. Pónce-Márquez. 2014a. Morphological and molecular characterization of Neoralfsia hancockii comb. nov. (Ralfsiales, Phaeophyceae) from topotype of San José del Cabo, Baja California, Mexico. Bot. Mar. 57: 139–146.
León-Alvarez, D., M.L. Núnez-Resendiz, and M.J. Wynne. 2014b.
Morphological and molecular studies on topotype material of Neoralfsia expansa (Phaeophyceae) reveal that Asian speci- mens assigned to this taxon are genetically distinct. Bot. Mar. 57: 351–358.
León-Tejera, H. and J. González-González. 1993. Macroalgas de Oaxaca. In: (S.I. Salazar-Vallejo and N.E. González, eds.) Biodiversidad marina y costera de México. Comisión Nacional de Biodiversidad y Centro de Investigaciones de Quintana Roo, México. pp. 486–498.
Lim P-E., M. Sakaguci, T. Hayunda, K. Kogame, S.M. Phang, and H. Kawai. 2007. Molecular phylogeny of crustose brown seaweeds (Ralfsiales, Phaeophyceae) inferred from rbcL sequences resulting in proposal for Neoralfsiaceae fam. nov. Phycologia 46: 456–466.
Lindauer, V.W. 1949. Notes on the marine algae of New Zealand. Pac.
Sci. 3: 246–248.
![]()
14 D. León-Alvarez et al.: Hapalospongidion gelatinosum, Hapalospongidiaceae fam. nov. ![]()
Poong, S.W. 2014. Taxonomy and phylogeny of crustose brown algae (Phaeophyceae) from Malaysia and Lombok Island, Indone-
sia. PhD. dissertation, Institute of Biological Sciences Faculty of Science, University of Malaya, Kuala Lumpur. Malaysia,
pp. 211–214.
Poong, S.W., P.-E. Lim, S.-M. Phang, G.S. Gerung, and H. Kawai. 2013. Mesospora elongata sp. nov. (Ralfsiales, Phaeophyceae), a new crustose brown algal species from the Indo-Pacific Region. Phycologia 52: 74–81.
Poong, S.W., P.-E. Lim, S.-M. Phang, J.A. West, and H. Kawai. 2014.
A molecular-assisted floristic survey of crustose brown algae (Phaeophyceae) from Malaysia and Lombok Island, Indone-
Weber-van Bosse, A. 1911. Notice sur quelques genres nouveaux d’algues de l’Archipel Malasien. Ann. Jard. Bot. Buitenzorg 24: 25–33.
West, J.A. and H.P. Calumpong. 1996. Mesospora negrosensis sp. nov. (Phaeophyta) from the Philippines. Philip. Sci. 33: 5–15. Woelkerling, W.J. 1988. The coralline red algae: an analysis of the
genera and subfamilies of non-geniculate Corallinaceae. British Museum (Natural History) and Oxford University Press, London and Oxford. pp. 157.
Womersley, H.B.S. 1987. The marine benthic flora of southern Aus- tralia. Part II. South Australian Government Printing Division, Adelaide. pp. 484.
sia based on rbcL and partial cox1 genes. J. Appl. Phycol. 26:
1231–1242.
Posada, D. and K.A. Crandall. 1998. Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818.
Saunders, De A. 1899. New or little-known brown algae of the Pacific coast. Erythea 7: 37–40, 1 pl.
Saunders, G.W. 2014. Long distance kelp rafting impacts seaweed biogeography in the Northeast Pacific: the kelp conveyor hypothesis. J. Phycol. 50: 968–974.
Serviere-Zaragoza, E. 1993. Descripción y análisis de la ficoflora
del litoral rocoso de Bahía de Banderas, Jalisco- Nayarit. Tesis doctoral, Fac. Ciencias, Universidad Nacional Autónoma de México, pp. 149.
Serviere-Zaragoza E., J. González-González, and D. Rodríguez- Vargas. 1993. Ficoflora de la región de Bahía de Banderas, Jalisco-Nayarit. In: (S.I. Salazar-Vallejo and N.E. González, eds.) Biodiversidad marina y costera de México. Comisión Nacional de Biodiversidad y Centro de Investigaciones de Quintana Roo, México, México, pp. 475–485.
Setchell, W.A. 1924. American Samoa: Part I. Vegetation of Tutuila Island. Part II. Ethnobotany of the Samoans. Part III. Vegetation of Rose Atoll. Publ. Carnegie Inst. Wash. 341: Pp. vi + 275.
Silberfeld T., J.W. Leigh, H. Verbruggen, C. Cruaud, B. de Reviers,
and F. Rousseau. 2010. A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): Investigating the evolutionary nature of the ‘‘brown algal crown radiation”. Molec. Phylogen. Evol. 56: 659–674.
Silva, P.C., P.W. Basson, and R.L. Moe. 1996. Catalogue of the
benthic marine algae of the Indian Ocean. Univ. Calif. Publ. Bot.
79: 1–1259.
Smith, G.M. 1944. Marine algae of the Monterey Peninsula. Stanford University Press, Stanford. pp. i–ix, 622.
Sophiammal Nettar, P. and M.V.N. Panikkar. 2009. Two new brown algal species from the family Ralfsiaceae (Ectocarpales, Phaeo- phyceae) from Kerala, India. Seaweed Res. Utiliz. 31: 7–10.
Stamatakis, A., A.J. Aberer, C. Goll, S.A. Smith, S.A. Berger, and F. Izquierdo-Carrasco. 2012. RAxML-Light: A tool for computing TeraByte phylogenies. Bioinformatics 28: 2064–2066.
Swofford, D. 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunder- land, Massachusetts.
Tanaka, J. and M. Chihara. 1982. Morphology and taxonomy of Mes- ospora schmidtii Weber van Bosse, Mesosporaceae fam. nov. (Ralfsiales, Phaeophyceae). Phycologia 21: 382–389.
Thiers, B. 2016. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Available at http://sweetgum.nybg.org/ih/. Accessed 5 Oct 2016.
Supplemental Material: The online version of this article
(DOI: 10.1515/bot-2017-0020) offers supplementary material, available to authorized users.

Daniel León-Alvarez
Departamento de Biología Comparada, Laboratorio de Ficología and Sección de Algas del Herbario de la Facultad de
Ciencias, Universidad Nacional Autónoma de México, UNAM, Ciudad Universitaria, Ciudad de México 04510, Mexico, dla@ciencias.unam.mx
Daniel León-Alvarez is an Associate Professor in the National Univer- sity of México and curator of algae in the Algae Section of the Sci- ences Faculty Herbarium (FCME). His research interests have been centered mostly on marine brown algae. His most recently published works are in Botanica Marina (2014), relative to the brown crustose algae. However, he works also with green and red marine algae. His books “Género de algas marinas tropicales de México” include the green (2007), brown (2012) and red algae (2017).

Viviana Patricia Reyes-Gómez Laboratorio de Ficología and Sección de Algas del Herbario de la Facultad de
Ciencias, Universidad Nacional Autónoma
de México, UNAM, Ciudad de México 04510, Mexico
Viviana P. Reyes-Gómez is a biologist from Universidad Nacional de Colombia and has a Master’s in Marine Science from Universidad Nacional Autónoma de México. At present she is a researcher col- laborator in the Herbarium Seaweed Section of the Faculty of Sciences, UNAM. Her research interests have been centered mostly on the bio- diversity of seaweeds from International Biosphere Reserve Seaflower and phylogenetics relationships of crustose brown algae from Mexico.

Michael J. Wynne
University of Michigan Herbarium, 3600
Varsity Drive, Ann Arbor, MI 48108, USA;
and Department of Ecology and Evolutionary Biologyand Herbarium, University of Michigan, Ann Arbor, MI 48109, USA
Michael J. Wynne is Professor Emeritus at the University of Michigan, Ann Arbor, and Curator of Algae, Emeritus, in the University of Michigan Herbarium. His research interests have been centered mostly on marine red algae. His book “The red algal families Delesseriaceae and Sarcomeniaceae” was published in 2014. He
has produced periodic revisions of his “A checklist of benthic marine algae of the tropical and subtropical Western Atlantic”, including
the recent publication of the fourth revision.

María Edith Ponce-Márquez
Laboratorio de Ficología and Sección de
Algas del Herbario de la Facultad de Ciencias, Universidad Nacional Autónoma de México, UNAM, Ciudad de México 04510, Mexico;
and Departamento de Biología Comparada, Taller Protistas y Algas, Facultad de Ciencias, Universidad Nacional Autónoma de México, Ciudad Universitaria, Coyoacán 04510, Ciudad de México, Mexico
María Edith Ponce Márquez is a Professor and academic technician in the National University of Mexico. Her Master‘s studies were on cytogenetics in red algae and her doctoral studies carried out in the molecular biology and cytogenetics of seaweeds. Her research inter- ests are in marine algae. She has collaborated in different projects
of investigation, mainly red algae and brown crustose algae. She is a co-author of the book “Género de algas marinas tropicales de México” (red algae, 2017).

Nataly Quiróz-González
Laboratorio de Ficología and Sección de Algas del Herbario de la Facultad de Ciencias, Universidad Nacional Autónoma
de México, UNAM, Ciudad de México 04510, Mexico
Nataly Quiróz-González is a biologist (Universidad Juárez Autónoma de Tabasco) and has a MSc from the Universidad Nacional Autónoma de México. Her area of research are marine algae, particularly red algae. Currently, she collaborates on different projects on taxonomy, diversity and ecology of marine algae.
![]()
![]() Botanica Marina 2017 | Volume x | Issue x
Botanica Marina 2017 | Volume x | Issue x
![]()
DOI 10.1515/bot-2017-0020
Botanica Marina 2017; x(x): xxx–xxx

Research article: Morphological and molecular evidence is provided to distinguish between Hapalospongidion and Mesospora and for the establishment of the new family Hapalospongidiaceae. A lectotype and an epitype for Hapalospongidion gelatinosum are designated.
Keywords: Hapalospongidiaceae fam. nov.; Hapalospongidion gelatinosum; Phaeophyceae; phylogeny, taxonomy.
![]()